Easy-to-use, pre-filled syringe
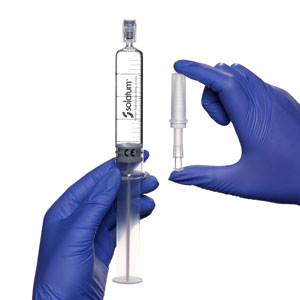
Remove pre-filled syringe of Solatum GAG Replenisher from sterile tray. Remove multi-fit luer-lock adapter from sterile pack (included).
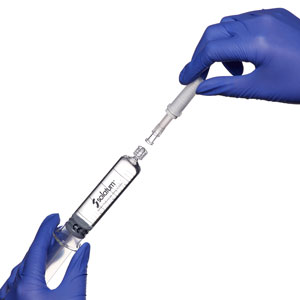
Attach multi-fit luer-lock adapter to pre-filled syringe
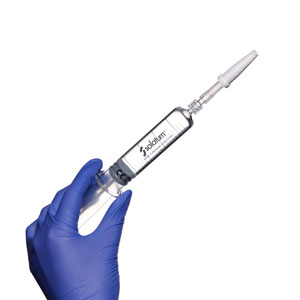
Expel any excess air from syringe. It is now ready to use
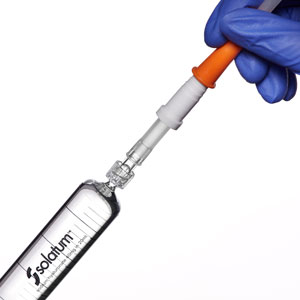
Attach to the pre-inserted urethral catheter and instil the solution into the bladder
Instructions for use
Solatum Sodium Hyaluronate GAG Replenisher for Urology Pre-filled Syringe with Luer-lock Adapter
Indication: For temporary replacement of the glycosaminoglycan (GAG) layer of the bladder.
Description: The glycosaminoglycan (GAG) layer on the luminal surface of the bladder wall is believed to provide a protective barrier against microorganisms, carcinogens, crystals and other agents present in the urine and has been identified as the primary defence mechanism in protecting the transitional epithelium from urinary irritants. Deficiencies in this GAG layer of the bladder epithelium may destroy its barrier function and allow the adherence of bacteria, microcrystals, proteins and ions, or the movement of ionic and nonionic solute residues (i.e. urea) across the epithelium. Solatum™ Sodium hyaluronate GAG replenisher has been developed to temporarily replenish the deficient GAG layer on the bladder epithelium. The active substance is a highly purified sodium salt of hyaluronic acid (HA) or hyaluronate.
Contents: Each Solatum™ prefilled syringe contains 40mg sodium hyaluronate in 20ml solution. Solatum™ is supplied with a separately packed sterile Luer-lock adaptor in the carton.
Directions: The patient should empty their bladder and have a urethral catheter inserted. Any residual urine should be drained. Connect the supplied luer-lock adapter (or other female luer-lock adapter) between the pre-filled syringe and pre-inserted urethral catheter (not supplied). Instill the entire volume of the Solatum™ solution into the bladder. Discard any unused portion. For best results, Solatum™ should be retained in the bladder for as long as possible (a minimum of 30 minutes), ideally up to 60 minutes.
Dosage Regimen Recommended: There is evidence that the GAG layer of the bladder is deficient in patients with chronic cystitis (interstitial cystitis, painful bladder, recurrent bacterial cystitis, radiation cystitis, cystitis associated with trauma). To alleviate cystitis associated with these conditions, it is recommended that Solatum™ be instilled into the bladder each week for 4 – 6 treatments and then monthly until symptoms resolve – usually 6 months in total. The patient should stop treatment and the patient and their Physician and/or Specialist Nurse should establish if symptoms have resolved. Treatment can be continued for longer on the decision of the Physician and/or Specialist Nurse.
Precaution: Do not administer to patients with known hypersensitivity reactions. Discontinue use if adverse reactions are experienced. As no clinical evidence is available on the use of hyaluronic acid in children, pregnant and lactating women, treatment with Solatum™ is not recommended in these patients.
Warning: For single use only. Discard after use.
Storage: Store at room temperature (2 – 25 °C). Do not freeze.
Supplied: 1 x 25ml Prefilled Syringe of Solatum™ 40mg. 1 x Primed Luer-Lock Adapter (CE 0482).
Date of Preparation: June 2019

© Solatum 2019